Greenhouse Gases
Introduction
Greenhouse gases are essential in making it possible for life to exist on Earth. Greenhouse gases are materials in the atmosphere which function in ways that retain heat energy. Without these gases much greater amounts of heat would be emitted from the land and oceans of the earth back into space, and the average temperature of the earth's surface would be much lower than now, approximately -18° C (0° F). Thus, greenhouse gases are critical factors in securing a habitable environment for almost all life forms on earth.
The principal greenhouse gases on Earth are:
- carbon dioxide
- methane
- nitrous oxide
- ozone
- water vapor
In addition, there are gases emitted in industrial processes that are powerful in their greenhouse effects. Among these gases are:
- Hydrofluorocarbons (HFCs)
- Perfluorocarbons (PFCs)
- Sulfur hexafluoride (SF6}
- Nitrogen trifluoride (NF3}
Although the temperature level provided by greenhouse gases is essential for most life on earth, substantial problems arise when the concentration of greenhouse gases becomes too high. In such cases the retention of heat becomes too intense, and the resultant rise in temperature becomes disruptive and in many cases threatening to both human life and the earth's ecosystems.
Greenhouse Effect
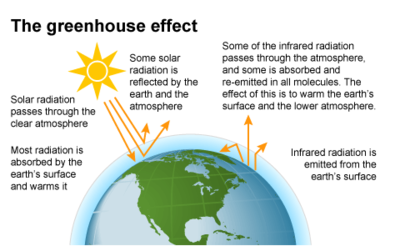
The greenhouse effect is the warming of the earth's atmosphere and surface due to added retention of heat from the sun by the action of certain gases in the atmosphere. This added retention of heat is the result of absorption by atmospheric greenhouse gases of heat emitted from the earth's surface and the re-emission of that heat back to the earth. This re-emission results in a net increase in the earth's temperature and actually makes life possible on earth. Were it not for the absorptive/re-emission action by greenhouse gases, earth's temperature would be in the range of 0 degrees Fahrenheit.
The natural course of the greenhouse effect has made life possible on earth for many millions of years. However, modern accelerated release of CO2 and other greenhouse gases into the atmosphere by human-caused (anthropogenic) burning of fossil fuels has been so massive that it has greatly increased the heat level and temperature of the earth. The release of excessive greenhouse gases has exceeded the capacity of natural processes to keep greenhouse gas levels at a moderate and consistent level and thus imperiled the stability of the earth's climate, raised the globe's temperature by potentially dangerous amounts, and possibly threatened the stability of human society.
Results of Greenhouse Effect
The Greenhouse Effect has many results reflected in changes in the earth and its systems. Some of these results are:
- warming of the atmosphere
- warming of the ocean
- changes in the global water cycle
- global reduction of snow and ice
- sea level rise
- increased acidification of the ocean
There is strong agreement among most climatologists and other researchers studying climate change that the results described above, as well as many others, are produced by human activities utilizing fossil fuels for the generation of energy.
Global CO2 Emissions and Atmospheric Concentrations
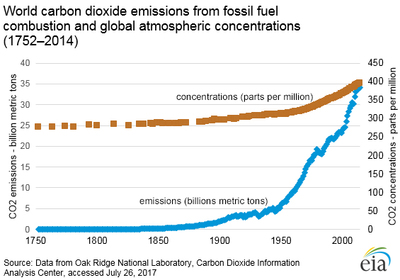
As the above chart shows, emission of carbon dioxide has risen steadily since the mid-1800's. This is due to the increasing use of fossil fuels by developed and developing countries. These uses have accelerated to the point where CO2 concentration in the atmosphere has increased from less than 300 ppm (parts per million) when the industrial revolution began around 1750, to over 409 ppm in 2018.
Although there are ways in nature where CO2 is absorbed from the atmosphere, for example photosynthesis, the human release of CO2 from fossil fuel use has overwhelmed the natural rate of absorption of CO2 and caused its atmospheric concentration to accelerate beyond what natural extraction of CO2 from the atmosphere can possibly accomplish.
Global Atmospheric Carbon Dioxide over Time
The two charts shown below demonstrate the contrast between modern levels of CO2 (chart on the right) and the levels of CO2 dating back several hundred thousand years (chart on the left). Note that even though the earth passed through numerous cycles of CO2 concentration, the difference of CO2 levels never came close to the extreme level of CO2 that we are presently experiencing. This extreme CO2 level can only be explained as a result of human use of fossil fuels.
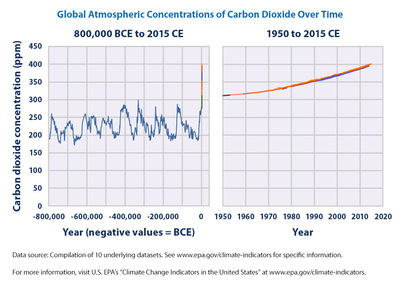
Global Atmospheric Concentrations of Carbon Dioxide Over Time
Source:EPA: Climate Change Indicators
US Greenhouse Gases 2016
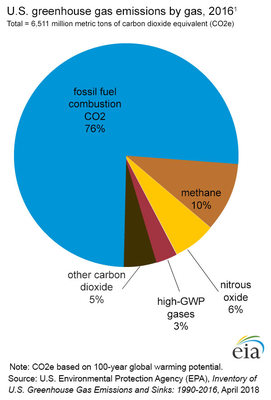
About 76% of greenhouse gas emissions in 2016 was CO2, released as a result of fossil fuel combustion. This was followed by methane at 10% and nitrous oxide at 6%. The sources of atmospheric methane are landfills, coal mines, agriculture, and industrial oil and natural gas operations. Nitrous oxide (N2O) comes from nitrogen fertilizers, burning fossil fuels, and certain industrial and waste management processes.
There are also anthropogenic industrial gases that have high global warming potential (GWP). These gases are:
- Hydrofluorocarbons (HFCs)
- Perfluorocarbons (PFCs)
- Sulfur hexafluoride (SF6)
- Nitrogen trifluoride (NF3)
The emission of the above industrial GWP gases are currently strictly regulated by international agreements. In addition to their potential as greenhouse gases, these gases are also, in many cases, capable of destruction of the stratospheric ozone layer, which protects life on Earth from damage by high energy ultra-violet (UV) radiation from the sun.
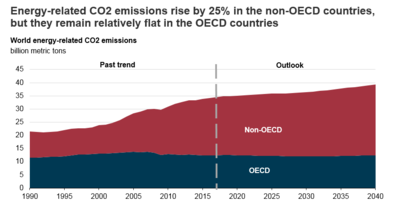
Projected Global CO2 Emissions
Without effective constraints on CO2 emissions, the amount of anthropogenic CO2 emitted from the present to 2040 is estimated to increase by approximately 16%. Most of that increase will be from non-OECD nations, which will be growing in their economies and will rely on fossil fuels to do so.
Ocean Acidification
The measure of acidity in chemical systems is the pH value. pH values can range from 0 to 14. Values in the range less than 7 are acidic, pH of 7 is neutral, and a pH greater than 7 is basic (alkaline). The oceans are normally slightly alkaline, with a pre-industrial pH value of approximately 8.179. The current pH value of the oceans is approx 8.07, which represents a decline in alkalinity in the last 2-3 centuries.
Increased ocean acidity is caused by increased concentrations of CO2 in the atmosphere, the result of massive combustion of fossil fuels for the generation of energy for modern civilizations. Atmospheric CO2 is absorbed by the oceans and in its reaction with water, releases hydrogen ions that lower ocean pH levels.
The chemistry of CO2 dissolved in ocean water results in a decline of carbonate ions, which causes a decrease in the availability of calcium carbonate, the primary constituent of shells and other exoskeletons of marine organisms. This is a major issue, threatening the viability of numerous ocean ecosystems and resulting in the marked decline of numerous marine species.